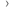Non-Hodgkin lymphoma risk
The estimated lifetime risk of being diagnosed with non-Hodgkin lymphoma is 1 in 71 (1%) for females, and 1 in 52 (2%) for males born in 1961 in the UK. [1]
These figures have been calculated on the assumption that the possibility of having more than one diagnosis of non-Hodgkin lymphoma over the course of a lifetime is very low ('Current Probability' method).[2]
See also
Lifetime risk for all cancers combined and cancers compared
Non-Hodgkin lymphoma incidence statistics
Want to generate bespoke preventable cancers stats statements? Download our interactive statement generator.
References
- Lifetime risk estimates calculated by the Cancer Intelligence Team at Cancer Research UK 2023.
- Estève J, Benhamou E, Raymond L. Statistical methods in cancer research. Volume IV. Descriptive epidemiology. IARC Sci Publ. 1994;(128):1-302.
About this data
Data is for UK, past and projected cancer incidence and mortality and all-cause mortality rates for those born in 1961, ICD-10 C15.
Calculated by the Cancer Intelligence Team at Cancer Research UK, 2023 (as yet unpublished). Lifetime risk of being diagnosed with cancer for people in the UK born in 1961. Based on method from Esteve et al. 1994 [2], using projected cancer incidence (using data up to 2018) calculated by the Cancer Intelligence Team at Cancer Research UK and projected all-cause mortality (using data up to 2020, with adjustment for COVID impact) calculated by Office for National Statistics. Differences from previous analyses are attributable mainly toslowing pace of improvement in life expectancy, and also to slowing/stabilising increases in cancer incidence.
Last reviewed: 14 December 2023
3% of non-Hodgkin lymphoma cases in the UK are preventable.[1]
See also
Want to generate bespoke preventable cancers stats statements? Download our interactive statement generator.
Learn how attributable risk is calculated
Hodgkin lymphoma risk statistics
References
- Brown KF, Rumgay H, Dunlop C, et al. The fraction of cancer attributable to known risk factors in England, Wales, Scotland, Northern Ireland, and the UK overall in 2015. British Journal of Cancer 2018.
Last reviewed: 14 June 2018
International Agency for Research on Cancer (IARC) classifies the role of this risk factor in cancer development.[1] 58% of MALT cases in the UK are caused by H. pylori infection, that's 2% of all NHL cases.[2]
Marginal zone lymphomas (MZL) risk is 1.6 times higher in people with an ulcer (used as a proxy measure for H. pylori infection), a pooled analysis showed.[3]
See also
Learn how attributable risk is calculated
View our health information on infections and cancer
Hodgkin lymphoma risk statistics
References
- Cogliano VJ, Baan R, Straif K, et al. Preventable Exposures Associated With Human Cancers. JNCI 2011;103(24):1827-39.
- Calculated by the Statistical Information Team at Cancer Research UK, 2018. Based on Brown KF, Rumgay H, Dunlop C, et al. The fraction of cancer attributable to known risk factors in England, Wales, Scotland, Northern Ireland, and the UK overall in 2015. British Journal of Cancer 2018.
- Bracci PM, Benavente Y, Turner JJ, et al. Medical history, lifestyle, family history, and occupational risk factors for marginal zone lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014;2014(48):52-65.
Last reviewed: 1 October 2018
International Agency for Research on Cancer (IARC) classifies the role of this risk factor in cancer development.[1] Less than 1% of NHL cases in the UK are caused by hepatitis C virus (HCV) infection.[2]
NHL cancer risk is 26% higher in people with HCV infection compared to individuals without the infection, a meta-analysis of cohort studies showed.[3] NHL patients are more than twice as likely to have HBV infection, compared with healthy controls, meta-analyses have shown.[4-6]
Marginal zone lymphomas (MZL) risk is 3 times higher in people with a history of HCV infection, a pooled analysis showed.[7] Diffuse large B-cell lymphoma (DLBCL) risk is 2 times higher in people with a history of HCV infection versus those without, a pooled analysis showed.[8]
See also
Learn how attributable risk is calculated
View our health information on infections and risk
Hodgkin lymphoma risk statistics
References
- Cogliano VJ, Baan R, Straif K, et al. Preventable Exposures Associated With Human Cancers. JNCI 2011;103(24):1827-39.
- Calculated by the Statistical Information Team at Cancer Research UK, 2018. Based on Brown KF, Rumgay H, Dunlop C, et al. The fraction of cancer attributable to known risk factors in England, Wales, Scotland, Northern Ireland, and the UK overall in 2015. British Journal of Cancer 2018.
- Zhu X, Jing L, Li X. Hepatitis C virus infection is a risk factor for non-Hodgkin lymphoma: A MOOSE-compliant meta-analysis. Medicine. 2019 1;98(11):e14755.
- Nath A, Agarwal R, Malhotra P, et al. Prevalence of hepatitis B virus infection in non-Hodgkin lymphoma: a systematic review and meta-analysis Intern Med J 2010;40:633-41.
- Dalia S, Chavez J, Castillo JJ, Sokol L. Hepatitis B infection increases the risk of non-Hodgkin lymphoma: a meta-analysis of observational studies. Leuk Res. 2013;37(9):1107-15.
- Yi HZ, Chen JJ, Cen H, et al. Association between infection of hepatitis B virus and onset risk of B-cell non-Hodgkin's lymphoma: a systematic review and a meta-analysis. Med Oncol 2014;31(8):84.
- Bracci PM, Benavente Y, Turner JJ, et al. Medical history, lifestyle, family history, and occupational risk factors for marginal zone lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014;2014(48):52-65.
- Cerhan JR, Kricker A, Paltiel O, et al. Medical history, lifestyle, family history, and occupational risk factors for diffuse large B-cell lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014;2014(48):15-25.
Last reviewed: 15 June 2022
International Agency for Research on Cancer (IARC) classifies the role of this risk factor in cancer development.[1] Less than 1% of NHL cases in the UK are caused by HIV infection.[2]
NHL risk is around 11 times higher in people with HIV, compared with the general population, a cohort study has shown.[3] Risk varies by NHL subtype; the risks of AIDS-defining NHL subtypes (including DLBCL) are 18+ times higher in people with HIV versus the general population, while the risks of non AIDS-defining NHL subtypes are increased to a lesser extent, a cohort study showed.[4] NHL risk in HIV-positive people increases with HIV severity, cohort studies have shown.[5,6]
HIV-associated NHL risk has decreased markedly since highly active antiretroviral therapy (HAART) was introduced to treat HIV in 1996,[7-11] probably because HAART improves immune system functioning.[12]
See also
Learn how attributable risk is calculated
View our health information on infections and cancer
Hodgkin lymphoma risk statistics
References
- Cogliano VJ, Baan R, Straif K, et al. Preventable Exposures Associated With Human Cancers. JNCI 2011;103(24):1827-39.
- Calculated by the Statistical Information Team at Cancer Research UK, 2018. Based on Brown KF, Rumgay H, Dunlop C, et al. The fraction of cancer attributable to known risk factors in England, Wales, Scotland, Northern Ireland, and the UK overall in 2015. British Journal of Cancer 2018.
- Seaberg EC, Wiley D, Martínez-Maza O, et al. Cancer incidence in the multicenter aids cohort study before and during the HAART era: 1984 to 2007. Cancer 2010;116:5507-16.
- Gibson TM1, Morton LM, Shiels MS, et al. Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: a population-based study. AIDS 2014 Sep;28(15):2313-8.
- Achenbach CJ, Buchanan AL, Cole SR, et al. HIV Viremia and Incidence of Non-Hodgkin Lymphoma in Patients Successfully Treated With Antiretroviral Therapy. Clin Infect Dis. 2014.
- Engels EA, Pfeiffer RM, Landgren O, et al. Immunologic and virologic predictors of AIDS-related non-hodgkin lymphoma in the highly active antiretroviral therapy era. J Acquir Immune Defic Syndr. 2010;54(1):78-84.
- Roman E, Smith AG. Epidemiology of lymphomas. Histopathology 2011;58:4-14.
- Boffetta PI. Epidemiology of adult non-Hodgkin lymphoma. Ann Oncol 2011;22:iv27-iv31.
- Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59-67.
- Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS 2006;20:1645-54.
- International Collaboration on HIV and Cancer. Highly Active Antiretroviral Therapy and Incidence of Cancer in Human Immunodeficiency Virus-Infected Adults. J Natl Cancer I 2000;92:1823-30.
- Palmieri C, Treibel T, Large O, et al. AIDS-related non-Hodgkin's lymphoma in the first decade of highly active antiretroviral therapy. QJM 2006;99:811-26.
Last reviewed: 1 October 2018
International Agency for Research on Cancer (IARC) classifies the role of this risk factor in cancer development.[1] Less than 1% of NHL cases in the UK are caused by Epstein-Barr virus (EBV) infection.[2]
NHL risk is 26% higher in people with previous infectious mononucleosis (which is caused by EBV), a pooled analysis showed.[3] NHL risk is higher in EBV-positive people, a meta-analysis showed.[4]
See also
Learn how attributable risk is calculated
View our health information on infections and cancer
Hodgkin lymphoma risk statistics
References
- Cogliano VJ, Baan R, Straif K, et al. Preventable Exposures Associated With Human Cancers. JNCI 2011;103(24):1827-39.
- Calculated by the Statistical Information Team at Cancer Research UK, 2018. Based on Brown KF, Rumgay H, Dunlop C, et al. The fraction of cancer attributable to known risk factors in England, Wales, Scotland, Northern Ireland, and the UK overall in 2015. British Journal of Cancer 2018.
- Becker N, Falster MO, Vajdic CM, et al. Self-reported history of infections and the risk of non-Hodgkin lymphoma: An InterLymph pooled analysis. Int J Cancer 2012:2342-2348.
- Teras LR, Rollison DE, Pawlita M, et al. Epstein-Barr virus and risk of non-Hodgkin lymphoma in the cancer prevention study-II and a meta-analysis of serologic studies. Int J Cancer. 2014. doi: 10.1002/ijc.28971.
Last reviewed: 1 October 2018
Immune dysregulation underpins both autoimmune disease and lymphoma development.
The NHL subtypes most often associated with autoimmunity are diffuse large B-cell lymphoma (DLBCL) and marginal zone lymphomas (MZL).[1-4]
Sjögren’s syndrome
NHL risk is 14-19 times higher in people with primary Sjögren’s syndrome, compared with the general population, a meta-analysis showed.[5]
Systemic lupus erythematosus
NHL risk is 5-7 times higher in people with systemic lupus erythematosus (SLE), compared with the general population, meta-analyses have shown.[5-7]
Rheumatoid arthritis
NHL risk is 2.3 times higher in people with rheumatoid arthritis, compared with the general population, a meta-analysis showed.[8]
NHL risk (B-cell NHL subtypes) in inflammatory rheumatic disease patients may be higher in those receiving antitumor necrosis factor alpha (anti-TNFα) treatment.[9,10]
Coeliac disease
NHL risk is 2-6-4.4 times higher in people with coeliac disease (enteropathy-associated T-cell lymphomas – EATCL/EATL) compared with the general population, meta-analyses have shown.[11,12]
Sclerosis
NHL risk is 2.7 times higher in people with systemic sclerosis, compared with the general population, a meta-analysis showed. [13]
Psoriasis
NHL risk is 40% higher in people with psoriasis, compared with the general population, a meta-analysis showed.[14]
See also
Learn how attributable risk is calculated
Hodgkin lymphoma risk statistics
References
-
Dias C, Isenberg DA. Susceptibility of patients with rheumatic diseases to B-cell non-Hodgkin lymphom a. Nat Rev Rheumatol 2011;7:360-8.
-
Ansell P, Simpson J, Lightfoot T, et al. Non-Hodgkin lymphoma and autoimmunity: Does gender matter? Int J Cancer 2011;129:460-6.
-
Anderson LA, Gadalla S, Morton LM, et al. Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. Int J Cancer 2009;125:398-405.
-
Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood 2008;111:4029-38.
-
Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: A meta-analysis. Arch Int Med 2005;165:2337-44.
-
Apor E, O'Brien J, Stephen M, Castillo JJ. Systemic lupus erythematosus is associated with increased incidence of hematologic malignancies: a meta-analysis of prospective cohort studies. Leuk Res 2014;38(9):1067-71.
-
Cao L, Tong H, Xu G, et al. Systemic lupus erythematous and malignancy risk: a meta-analysis. PLoS One. 2015 Apr 17;10(4):e0122964.
-
Simon TA, Thompson A, Gandhi KK, et al. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015 Aug 15;17:212.
-
Wong A, Kerkoutian S, Said J, et al. Risk of lymphoma in patients receiving antitumor necrosis factor therapy: a meta-analysis of published randomized controlled studies. Clin Rheumatol 2012;31:631-6.
-
Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis 2011;70:1895-904.
-
Kane E, Newton R, Roman E. Non-Hodgkin lymphoma and gluten-sensitive enteropathy: estimate of risk using meta-analyses. Cancer Cause Control 2011;22:1435-44.
-
Tio M, Cox MR, Eslick GD. Meta-analysis: coeliac disease and the risk of all-cause mortality, any malignancy and lymphoid malignancy. Aliment Pharm Ther 2012;35:540-51.
-
Zhang JQ, Wan YN, Peng WJ, et al. The risk of cancer development in systemic sclerosis: a meta-analysis. Cancer Epidemiol. 2013;37(5):523-7.
-
Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013 Aug;27 Suppl 3:36-46.
Last reviewed: 1 October 2018
International Agency for Research on Cancer (IARC) classifies the role of this risk factor in cancer development.[1]
Trichloroethylene
NHL risk is higher in people with occupational exposure to trichloroethylenemeta-analyses have shown.[2-5]
Pesticides
NHL risk is 40% higher in people exposed to organochlorine pesticides and 22% higher in people exposed to organophosphate pesticides two meta-analyses showed.[6-7] NHL risk is 26% higher in people who have ever worked as a field crop/vegetable farm worker and 19% higher in people who have ever worked as a general farm worker a pooled analysis showed.[8]
See also
Learn how attributable risk is calculated
Hodgkin lymphoma risk statistics
References
- Cogliano VJ, Baan R, Straif K, et al. Preventable Exposures Associated With Human Cancers. JNCI 2011;103(24):1827-39.
- Scott CS, Jinot J. Trichloroethylene and cancer: systematic and quantitative review of epidemiologic evidence for identifying hazards. Int J Environ Res Public Health 2011;8:4238-72.
- Mandel JH, Kelsh MA, Mink PJ, et al. Occupational trichloroethylene exposure and non-Hodgkin’s lymphoma: a meta-analysis and review. Occup Environ Med 2006;63:597-607.
-
Karami S, Bassig B, Stewart PA, et al. Occupational trichloroethylene exposure and risk of lymphatic and haematopoietic cancers: a meta-analysis. Occup Environ Med. 2013;70(8):591-9.
-
Cocco P, Vermeulen R, Flore V, et al. Occupational exposure to trichloroethylene and risk of non-Hodgkin lymphoma and its major subtypes: a pooled InterLymph [correction of IinterLlymph] analysis. Occup Environ Med. 2013;70(11):795-802.
-
Luo D, Zhou T, Tao Y. Exposure to organochlorine pesticides and non-Hodgkin lymphoma: a meta-analysis of observational studies. Scientific Reports 2016;6(1).
-
Hu L, Luo D, Zhou T. The association between non-Hodgkin lymphoma and organophosphate pesticides exposure: A meta-analysis. Environmental Pollution 2017;231:319-328.
-
‘t Mannetje A, De Roos A, Boffetta P, et al. Occupation and Risk of Non-Hodgkin Lymphoma and Its Subtypes: A Pooled Analysis from the InterLymph Consortium. Environmental Health Perspectives 2016;124(4):396-405.
Last reviewed: 2 May 2019
NHL risk is higher in people with a first-degree relative (parent, sibling, child) with NHL, a pooled analysis and cohort study have shown;[1,2] relatives may develop the same NHL subtype.[3]
NHL risk may be higher in people with a first degree relative with other cancer types, particularly haematological cancers, pooled analyses of case-control studies have shown.[4-9]
Self-reported family history information and changes over time in NHL classification may impact on the reliability of this evidence.
See also
Learn how attributable risk is calculated
Hodgkin lymphoma risk statistics
See more information on how inherited genes can be a cause of cancer
References
- Wang SS, Slager SL, Brennan P, et al. Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma (NHL): a pooled analysis of 10 211 cases and 11 905 controls from the International Lymphoma Epidemiology Consortium (InterLymph). Blood 2007;109:3479-88.
- Crump C, Sundquist K, Sieh W, et al. Perinatal and Family Risk Factors for Non-Hodgkin Lymphoma in Early Life: A Swedish National Cohort Study J Natl Cancer I 2012;104:923-30.
- Goldin LR, Björkholm M, Kristinsson SY, et al. Highly increased familial risks for specific lymphoma subtypes. Brit J Haematol 2009;146:91-4.29.
- Zhang Y, Wang R, Holford T, et al. Family history of hematopoietic and non-hematopoietic malignancies and risk of non-Hodgkin lymphoma. Cancer Cause Control 2007;18:351-9.
- Negri E, Talamini R, Montella M, et al. Family History of Hemolymphopoietic and Other Cancers and Risk of Non-Hodgkin's Lymphoma. Cancer Epidem Biomar 2006;15:245-50.
- Smedby KE, Sampson JN, Turner JJ, et al. Medical history, lifestyle, family history, and occupational risk factors for mantle cell lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014;2014(48):76-86.
- Bracci PM, Benavente Y, Turner JJ, et al. Medical history, lifestyle, family history, and occupational risk factors for marginal zone lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014;2014(48):52-65.
- Linet MS, Vajdic CM, Morton LM, et al. Medical history, lifestyle, family history, and occupational risk factors for follicular lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014;2014(48):26-40.
- Cerhan JR, Kricker A, Paltiel O, et al. Medical history, lifestyle, family history, and occupational risk factors for diffuse large B-cell lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014;2014(48):15-25.
Last reviewed: 1 October 2018
Statistics by cancer type
View our latest cancer statistics including key stats, in-depth explanations and raw data on cancer incidence, mortality, survival, risk, and diagnosis and treatment.
Citation
You are welcome to reuse this Cancer Research UK content for your own work.
Credit us as authors by referencing Cancer Research UK as the primary source. Suggested styles are:
Web content: Cancer Research UK, full URL of the page, Accessed [month] [year].
Publications: Cancer Research UK ([year of publication]), Name of publication, Cancer Research UK.
Graphics (when reused unaltered): Credit: Cancer Research UK.
Graphics (when recreated with differences): Based on a graphic created by Cancer Research UK.
When Cancer Research UK material is used for commercial reasons, we encourage a donation to our life-saving research.
Send a cheque payable to Cancer Research UK to: Cancer Research UK, 2 Redman Place, London, E20 1JQ or
Newsletter
Stay up to date by signing up to our cancer statistics and intelligence newsletter
Acknowledgements
We are grateful to the many organisations across the UK which collect, analyse, and share the data which we use, and to the patients and public who consent for their data to be used. Find out more about the sources which are essential for our statistics.