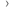This diagram shows the process that researchers follow to set up and run a clinical trial. There is more information about each of the stages below.
Having an idea
Researchers or doctors have an idea of something they would like to test. This could come from laboratory work, other trial results or experience with patients. The idea could involve:
- a new treatment
- a different combination of treatments
- using an existing treatment for a different condition
- testing a new way of giving an existing treatment
Writing the plan
The team interested in doing the trial write a detailed plan (protocol) that everyone involved in the trial will use. It contains lots of information, including:
- why they want to do the trial
- who will be able to take part (the eligibility criteria)
- how many people they need to take part
- what the treatments are
- what tests and appointments people will have before, during and after treatment
- how and when they plan to analyse the results
Peer review
Doctors, researchers and statistics experts who are not involved with the trial then check the protocol to make sure it is ok. They look at all aspects of the trial including:
- how important the research question is
- how easy it will be to find people to take part
- whether they plan to analyse the results in the right way
- if there are any possible difficulties the research team have not thought of
Funding
The research team will need money to run the trial. This may come from a charity, the government or a pharmaceutical company. We have information about
how trials are funded.
Sponsor
All trials should have a sponsor. The sponsor is usually an organisation or it can be an individual. Some trials might have more than one sponsor and these are called co-sponsors. The sponsor is responsible for the overall management of the trial. This includes making sure that the trial team follows research regulations. And that the results are analysed and reported properly.
Ethical approval
The protocol must be approved by an ethics committee. They must make sure it is in the best interest of the patients and will be possible to run. You can read more about
how trials are approved.
Hospitals take part
Once the trial has been given the go ahead, hospitals with the right expertise and equipment can sign up so they can recruit patients to the trial. The staff involved will usually have some training from the research team.
Patients join the trial
All trials have entry conditions called
eligibility criteria 
. Once the trial is up and running, patients who fit the criteria can take part.
The research team must give everyone who wants to take part all the information they need. This includes what is involved and all the possible
advantages and disadvantages.
Once they have read the information and asked any questions they have, they sign a consent form to say they are happy to join the trial. We have information about
what the trial team should tell you.
Treatment
The people taking part can start treatment once they have been given all the information they need and signed a consent form.
Depending on the design of the trial, they may be put into one of the treatment groups at random. This makes sure the different trial groups are even and means the results are more reliable. You can read about what a
randomised trial is.
Analysis of the results
Once everyone taking part has had treatment, the research team will look at and analyse the results. They will then draw some conclusions about the treatments in the trial. And they may make recommendations for future research.
Many research teams publish the results in a medical journal or present them at a conference. This is to share what they have learnt.
Sometimes the results show that the new treatment isn't better than the existing treatment. Even so, it adds to our knowledge and understanding of cancer and how to treat it. The results may also help decide what should be done in the next trial.
Licensing new treatments
If the results of the trial show a new treatment is better than the existing treatment, it may be licensed. This is so that doctors can prescribe it. Licensing new treatments is quite a long and complicated process. It often depends on the results of more than one trial.
Data monitoring committee
The
data monitoring committee 
(DMC) is group of people who are not directly involved with the trial. They keep an eye on how things are going all the way through, and make sure everything is running safely. They change parts of the trial, or even stop the trial, if they feel they need to.
This page is due for review. We will update this as soon as possible.
References
Oxford Handbook of Clinical and Healthcare Research (1st edition)
R Sumantra, S Fitzpatrick, R Golubic and others
Oxford University Press, 2016
Related information
We have information about
Next review due: 1 February 2025
 . Once the trial is up and running, patients who fit the criteria can take part.
. Once the trial is up and running, patients who fit the criteria can take part.  (DMC) is group of people who are not directly involved with the trial. They keep an eye on how things are going all the way through, and make sure everything is running safely. They change parts of the trial, or even stop the trial, if they feel they need to.
(DMC) is group of people who are not directly involved with the trial. They keep an eye on how things are going all the way through, and make sure everything is running safely. They change parts of the trial, or even stop the trial, if they feel they need to.